2023 Q4 update: settling in
A wise friend recently asked, delicately, whether the shiny excitement of a new venture had worn off perhaps exposing a substrate of drudgery and doubt. On the contrary, after two years of rapid and continuous progress, a growing team and network provide a new kind of sustainable energy.
FINANCIAL
Early investors demonstrated their confidence in our mission and capability by funding a $150,000 SAFE round earmarked for legal and regulatory consulting services.
R&D continues with non-dilutive funding from the Maryland Industrial Partnerships (MIPS) $90,000 Phase 1 grant.
REGULATORY & IP
Regulatory consultant Mary Wentorf of Springboard Medical Device Consulting LLC was engaged to guide FDA clearance strategy and communications.
Intellectual property has been substantially strengthened with four patent filings in October and another big one in the works for a major connector innovation.
BUSINESS DEVELOPMENT
Attending The Medtech Conference in Anaheim as a member of the Maryland Commerce delegation offered connections with industry experts at all stages of development and scale.
Competing with medtech ventures from around the world, Djit was selected to join the Creative Destruction Lab (CDL) business accelerator in Vancouver, BC offering rich mentor relationships, market research support, and access to investor networks.
Relocating Djit’s main office to The LaunchPort, a medtech venture center in Baltimore, connects Djit with a community of innovators and funding opportunities.
Over the next few months, we will stay focused on de-risking a Seed Round planned for next year. Milestones in R&D, regulatory pathway, market validation, and hardening the IP portfolio will demonstrate a solid business case for investment.
ASK: Non-dilutive funding to accelerate R&D toward animal testing and manufacturing is a hot priority. If you have contacts at TEDCO or other funded research programs please tell them about us! We believe Djit is an ideal candidate for economic development initiatives and can provide ample evidence to support that assertion.
2023 Summer update: team building
Hello friends, advisors, collaborators, and investors.
The startup landscape is carpeted with carcasses of well-intentioned projects led by intelligent and hard-working founders. Those that make it across the first barren plain know that it takes a team. Djit benefitted hugely from I-Corps, LifeX Accelerator in Pittsburgh and Maryland’s TEDCO and UpStart community.
Nonetheless after getting as far as possible with preliminary design and market research, we found ourselves in a chicken/egg loop; needing technical development to de-risk investment AND needing to engage internal expertise without funding to avoid issues that would diminish long-term value. Serendipity and old networks came through for us.
In June 2023 Fraser Smith, Ph.D., assumed the role of Chief Innovation Officer, taking charge of R&D and intellectual property. Fraser has spent his career at Sarcos Inc. and its various incarnations. As a co-founder of Sarcos, former President of Raytheon-Sarcos, and President/CEO of Raytheon’s spinout Sarcos Corp., Fraser has been responsible for winning contracts and developing innovative mechanical engineering solutions for defense, entertainment, and medical device industries. With over 150 patents and many more in the works, including exciting new innovations for Djit, Fraser’s experience in value creation is a huge asset for Djit and our investors.
Fraser and I are high school classmates who share a deep intellectual curiosity and commitment to excellence forged in the challenging academic environment at Phillips Exeter Academy. Co-founder Dr. Ryan Katz fits right in to this culture. As a graduate of Duke and the University of Maryland Medical School, resident at Johns Hopkins, and fellow at the Curtis National Hand Center, Dr. Katz now teaches young surgeons in that fellowship program and is nationally recognized as a key opinion leader among hand surgeons. My role is to bring entrepreneurial experience along with design skills, relentless drive and a patient’s perspective. The core team has the capability to build a valuable IP portfolio and regulatory strategy in preparation for a Seed Round next year.
Ask
Our next goal is to add depth on the financial side. We need to recruit a Chief Financial Officer and a brilliant Board of Directors with strong networks in venture capital and private equity. A family history of advanced hand arthritis would be a plus!
Other News
In the meantime, here’s a recap of recent accomplishments and activities:
LifeX Greenhouse Accelerator - $20,000 award
TEDCO Network Advisor - Abba Poliakoff, Esq.
TEDCO Loaned Executive $6,000 award – Cyrus Etemad-Moghadam, RPM Tech, prototype fabrication
Maryland Industrial Partnership (MIPS) $90,000 award – Dr. Hugh Bruck, Ph.D. UM College Park
Maryland Department of Commerce scholarship to The Medtech Conference, Anaheim CA October 2023 - $2,500 award
Corporate conversion to Maryland C-Corp with IR Code Sect. 1202 qualification
Woman Owned Small Business (WOSB) Certification
Launchport / Danae - manufacturing resources; informal relationship
Friends and family SAFE Round in progress - $100,000 total raise
Stay in touch
You can reach me at 667-444-9322 or by email at marcia.hart@djit-medtech.com
Thank you again for your interest and support.
All the best,
— Marcia
Marcia Hart
Co-founder/CEO
2023 Q1 Update – Message from the CEO
Hello friends, advisors, and collaborators.
We've come a long way in a year. Your support and encouragement has made it possible to get to this point. Some highlights:
Filed provisional and nonprovisional patent applications for a small-joint implant device
Validated $4.2B market, regulatory pathway, reimbursement, and competitive landscape through intense commercialization research with NSF I-Corps and LifeX life sciences accelerators
Awarded $20,000 first prize from LifeX Labs pitch competition
Learned a ton about grants and grant writing
Recruited a powerful informal advisory board and engineering team ready to go on R&D
Now we need to get into the lab and validate the technology. We are looking for $50k - $125k, ideally in non-dilutive funding through economic development and foundation grants or competitions plus a small private raise to bridge timing gaps. Let me know if you are interested in learning more about this pre-seed round that will offer a risk premium.
Honor Roll
So many people have helped make this quest a reality. When I found out after my table saw accident that joint replacement for advanced arthritis in fingers is rare and risky due to deficiencies in current technology, I got motivated. My excellent hand surgeon, Dr. Ryan Katz at the Curtis National Hand Center in Baltimore, was equally inspired so we partnered up to address this huge unmet need.
Thank you, thank you, THANK YOU to all who have contributed. We would not be this far along without you, including all the surgeons we interviewed and many others not listed below.
Allan C. Doyle
Deb Hemingway
Andrew Abadeer, MD
Nicholas Calotta, MD
Grady White, Esq.
Abba Poliakoff, Esq.
NSF I-Corps UMD team
LifeX Accelerator cohort
Kelly Collier
Laura Ohlund
Glenn Watson
Michele Miguiolo
Julie Gulick
Rishaan Sharma
David H. Gibel
Sadaf Akbari
Alexandre Cavalca
Hugh Bruck, Ph.D.
Cyrus Etemad-Moghadam
Ben McCandless
Billy Petzold
Ed Rothstein
... and many more
Looking back...
It's encouraging to be reminded of how much can be accomplished with hard work and great support. [image]
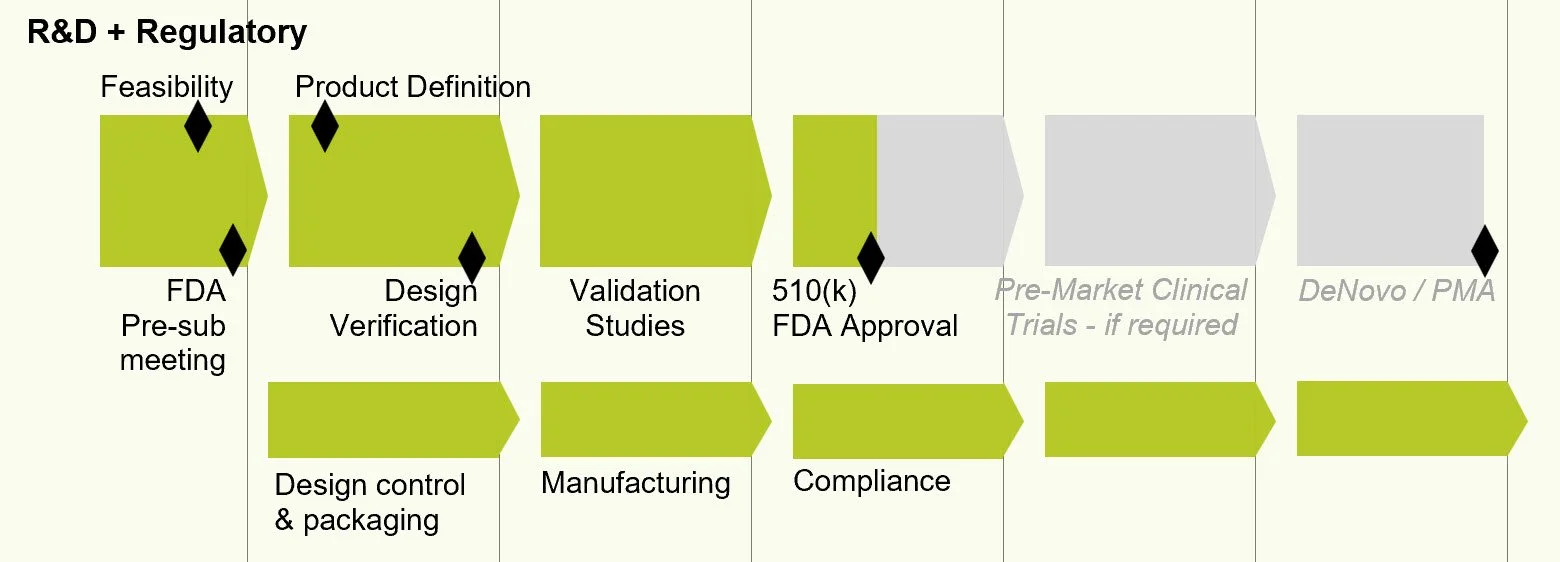
The work ahead
The years ahead are all about R&D and regulatory compliance for FDA approval. While business operations demand attention, critical activity will be in the lab. Grant writing and fundraising goes with the territory. We'll expand the team along the way. If you know of people who might want to join us, I would welcome your introduction. We're going to need grant writing, R&D, regulatory, business planning and market expertise. [image]
That's it for now. Please contact me with any questions or comments. I'd be happy to share more detail with you. My inbox is open for ideas, investment queries, introductions, and general encouragement.
Thanks, everyone. Let's stay in touch.
Gratefully,
Marcia Hart, CEO
mobile: 667-444-9322
Djit Medtech, LLC